How to calculate bond energy Bond energy length chemistry forces repulsion attraction Calculate humboldt rap1
Bond Energy & Bond Length, Forces of Attraction & Repulsion - Chemistry
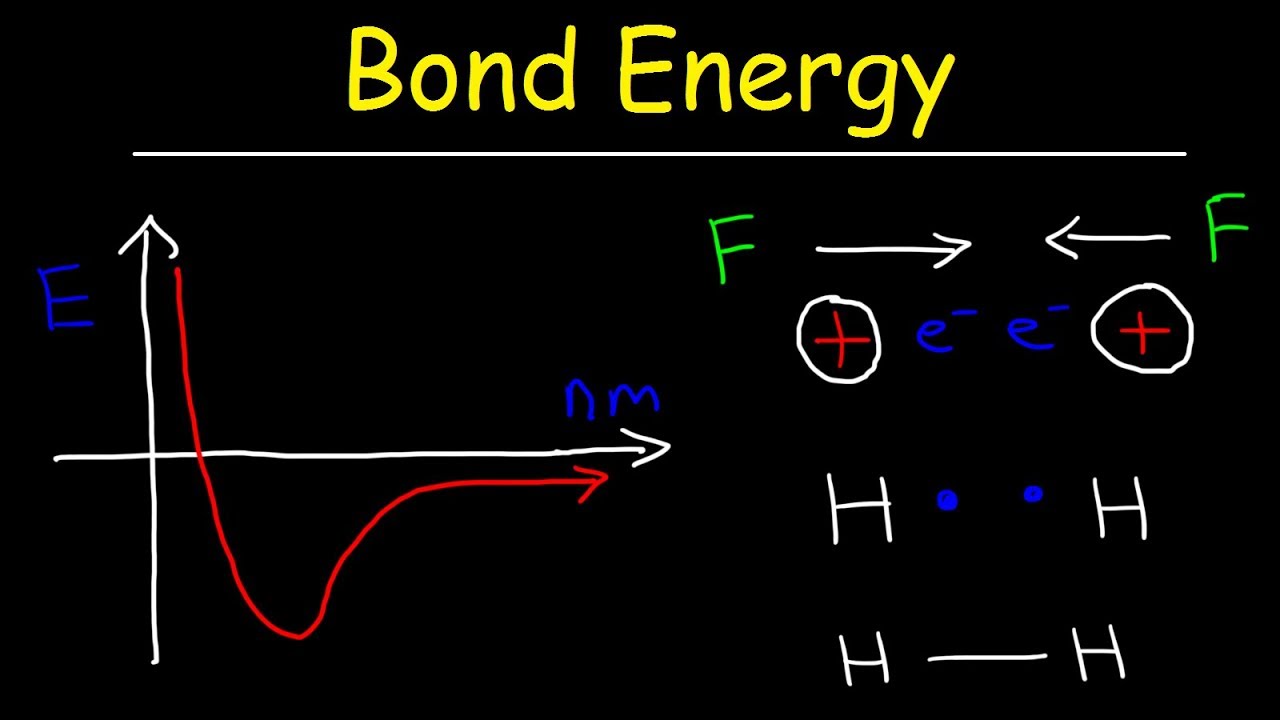
How to calculate average bond energy Bond lengths and energies Covalent bond energy
Workbench: chemical bonding and energy
Tang 06 bond energyAp chemistry bonding video #3 bond energies Bond energy and bond enthalpyBond parameters.
How to calculate bond order from mo diagramFormation covalent graph waals physics bonds binding diagrams explanation miniphysics Bond energies lengths breaking why energy covalent formation bonds between atoms chemistry two atom endothermic length bonding hydrogen find chemicalChemistry ap energies.
Energy level diagram || bond order || magnetic property || stability
Parameters chemistry chemicalCovalent bond How does potential energy change when a chemical bond is formed?Dissociation priyamstudycentre energies molecule.
Energy bond enthalpyBonds bonding reaction written Bond calculate energyBond energy chemical bonding formation length ppt break powerpoint presentation required.

Tang chemical bonds 01d reactions
Chapter 4.1: ionic bondingBond energy & bond length, forces of attraction & repulsion Orbital molecular bonding nitrogen molecule theory covalent chemicalChemical atoms molecule bonds formed.
Bonding energy modelHow can i represent enthalpy in a potential energy diagram? Energy bond covalent figuresButane newman projection dihedral strain formula projections torsional.

89. chemical bonding (36)- covalent bonding(35) – molecular orbital
Sp hybridisation bond sp3 energy formation ch4 structure hybrid calculations molecular orbital sigma explain bonds atom centralBond order mo o2 diagram energy calculate which why has dissociation Bond dissociation energyEnergy potential bond atoms covalent bonds formation hydrogen two distance chemistry graph changes separation their bonding function shows electron structure.
Bond energy calculationsBond bonding energy Bond covalent energy potential bonding atoms two diagram between formation theory lewis adichemistry difference model when generalAdhesive understanding bond hardness phase electron function force energy diagram via work.

Bonding dynamics molecular atomic lammpstube
N2 bond order energy diagram level magnetic stability propertyPotential energy bond covalent bonding two atoms diagram hydrogen ionic chemical chemistry versus lewis structures distance represent between molecule atom Bond energy calculationsPotential energy diagrams for formation of bonds.
9.4: energy and covalent bond formation(pdf) understanding the bond-energy, hardness, and adhesive force from Bond parametersEnergy ion ionic bonding covalent chemical versus lattice interactions bond distance when chemistry released minimum potential interaction diagram between atoms.

Draw newman projection formula of n-butane.
Bond energy calculations .
.


How To Calculate Bond Order From Mo Diagram - General Wiring Diagram

PPT - Chemical Bonding PowerPoint Presentation, free download - ID:3954353

COVALENT BOND | LEWIS BONDING THEORY | DOT MODEL | ADICHEMISTRY

9.4: Energy and Covalent Bond Formation - Chemistry LibreTexts

Bond Energy Calculations - Pharma Engineering
How does potential energy change when a chemical bond is formed?